
A drug used to treat Ebola patients has shown “very promising” early results in a trial of people admitted to hospital with coronavirus, scientists said.
Patients given remdesivir had a recovery time that was almost a third faster than those given a placebo, the first results from an international clinical trial showed.
Preliminary results also suggested a survival benefit, with a lower mortality rate of 8% for the group receiving the drug, compared with 11.6% for the placebo group, the National Institute of Allergy and Infectious Diseases (NIAD) said.
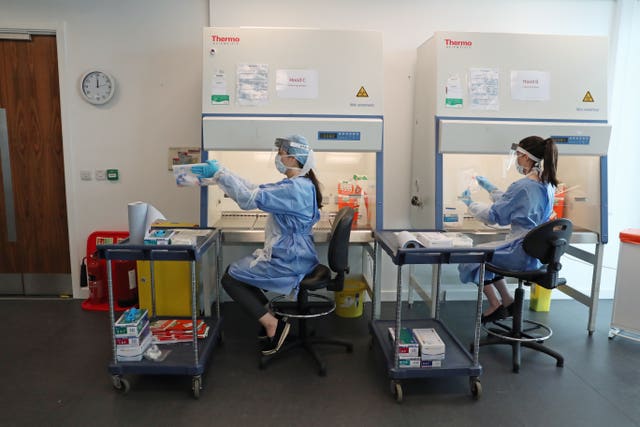
More than 1,000 patients have been recruited across the world, including 46 from the UK, for the Adaptive Covid-19 Treatment Trial, which began at the start of April.
Scientists involved in the study defined recovery as a patient being well enough to come off oxygen, being discharged from hospital or even returning to normal activity levels.
During the trial, which involved more than 70 hospitals across the globe, patients were given the antiviral drug every day for 10 days while they remained in hospital.
Professor Mahesh Parmar, director of the Medical Research Council Clinical Trials Unit at UCL, who oversaw the EU portion of the trial, said scientists will continue to gather further data while the early results are reviewed by regulators.
Prof Parmar said: “These results are very promising indeed. They show that this drug can clearly improve time to recovery.
“Before this drug can be made more widely available, a number of things need to happen: the data and results need to be reviewed by the regulators to assess whether the drug can be licensed and then they need assessment by the relevant health authorities in various countries.
“While this is happening, we will obtain more and longer term data from this trial, and other ones, on whether the drug also prevents deaths from Covid-19.”
Professor Sarah Pett, professor of infectious diseases at the UCL trials unit, paid tribute to the co-operation between teams as they worked to deliver results quickly.
She said: “Delivering the results of the first stage of the ACTT study in such a short time is down to the dedication and hard work of the teams in the USA, at UCL, all of the hospitals who participated in this trial, and of course most importantly, the patients who agreed to participate.
“The spirit of collaboration has never been stronger, in this time of urgent global need. The reward for all of us has been to provide data which suggests remdesivir helped the patients in the ACTT study recover more quickly. This is a foundation on which to build, and these findings will help other patients with Covid-19.”
A separate, smaller study in the Chinese city where coronavirus was first detected, found the drug did not speed up recovery or reduce deaths in people with Covid-19 compared with placebo in hospitalised patients.
That study, published in The Lancet on Wednesday, involved 237 adults from 10 hospitals in Wuhan.
But the authors said because the trial was stopped early, the true effectiveness of remdesivir remains unclear.
Professor Bin Cao, from China-Japan Friendship Hospital and Capital Medical University in China, who led the research, said it was not the outcome his team hoped for but recognised they were limited in the patients they could enrol.
The data safety monitoring board stopped the trial because researchers were unable to recruit enough patients following the steep decline of cases in China.
Remdesivir, which was originally developed to treat Ebola, is one of a handful of experimental drugs undergoing clinical trials worldwide to treat coronavirus.
Trudie Lang, professor of global health research at the University of Oxford, said the Chinese study being stopped showed the “limited window” of when there were enough patients to carry out clinical research.


Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here